What are CNFs? |
||||||||||||||||||||||
|
In recent years, demand for biodegradable, plant-based,
composite, also called "green composites" or
"biocomposites", is increasing in various sectors especially
the automotive sector of industry. Various kinds of natural
fibers have been used in green composites; such
reinforcements can be classified into two categories of
plant and animal fibers. Plant fibers may be produced from
different parts of the plants, such as seed, leave, skin,
bast, and fruit. Examples of such fibers are cotton, jute,
kenaf, coir, and flax. Examples of animal fibers include
silk, sinew, wool, catgut, angora, mohair and alpaca.
Natural fibers have lower mechanical properties as compared
to carbon and glass fibers. As a result, the current breed
of two biocomposites suffers from the twin drawbacks of
lower strength and fatigue properties compared with the
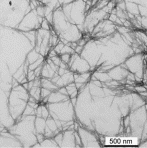
carbon or glass-fiber based polymer composites. Cellulose nanofibers (CNFs) (c.f. Fig-1), a new type of nanofibers made purely of cellulose molecules, have very good mechanical property compared to other natural fibers, and even carbon or glass fibers. Recent studies showed that the films or "nanopaper" of CNFs are the strongest man-made, cellulose-based materials. However, the full reinforcing potential of these materials has yet to be realized partly because of issues related to manufacturing processes. Recently, cellulose nanofibers have begun receiving serious consideration as potential reinforcement materials. The big problem with using CNF fibers was the energy requirements for breaking down cellulose fibers in nanofibers were prohibitively high until recently. Recent advances in chemical and mechanical technologies have drastically reduced the energy requirements for producing cellulose nanofibers. For this project, CNFs are provided by FPL produced at their pilot facility (c.f Fig-2). |
||||||||||||||||||||||
|
|
||||||||||||||||||||||
| Fig-1: (a-left) Schematic of CNFs;
(b-right) a transparent CNF film (Photos courtesy: Dr. Ron Sabo, Forest Products Laboratory) |
||||||||||||||||||||||
 |
||||||||||||||||||||||
| Fig-2: CNF production pilot plant
located at Forest Products Laboratory, USDA (Photos courtesy: Dr. Ron Sabo, Forest Products Laboratory) |
||||||||||||||||||||||
Why do we Need to Hydrophobize CNFs |
||||||||||||||||||||||
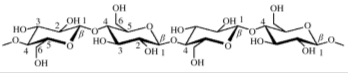
| Each repeating unit of CNF has 3 hydroxyl groups which make CNF highly hydrophilic. This high degree of hydrophilicity makes CNF incompatible with hydrophobic polymers such as epoxy. Hence there is a critical need for hydrophization of CNFs. In this study, we aim to hydrophobize CNFs using silylation technology. | ||||||||||||||||||||||
 |
||||||||||||||||||||||
| Structure-1: CNF backbone
showing the position of hydroxyl groups [Garner et al., Journal of Adhesion Science and Technology, 2008] |
||||||||||||||||||||||
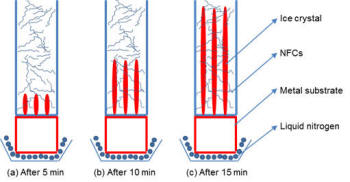
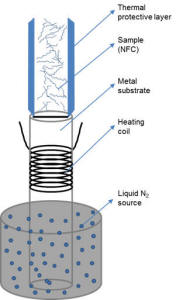
Description of Lyophilization Process |
||||||||||||||||||||||
|
 |
 |
| Making Random and Unidirectional Hydrophobic Porous Cellulose Nanofiber Structures | |